Nearly two years after the emergence of the COVID-19 pandemic, Israel may soon finally see its own homegrown vaccine become commercially available.
The jab will be a very late newcomer — lagging behind the first wave of COVID vaccines by almost a year — but its backers believe that it will find its rightful place in the global vaccine market, and may even prove in the long run to be more effective than existing jabs against coronavirus variants.
These beliefs were offered this month by Dr. Jonathan Javitt, chairman of NRx Pharmaceuticals, the American-Israeli clinical-stage pharmaceutical company tapped two months ago by the Israeli Defense Ministry to manufacture and market the country’s vaccine developed by the government-run Israel Institute for Biological Research (IIBR) in Ness Ziona.
“The vaccine deserves to be commercialized for the world. I believe it will find its place in the market,” Dr. Javitt told The Times of Israel recently. “The reality of COVID is that it keeps changing. There are new variants and we need to stay ahead of them. Otherwise, people will keep dying.”
The pandemic has already claimed the lives of over 4.5 million people worldwide.
The Israeli jab, dubbed BriLife, is currently in Phase IIb/III trials in Georgia with unvaccinated volunteers over the age of 18, and will soon begin to be administered in Ukraine, another a country with low vaccination rates.
The study is part of a non-inferiority trial with an already-approved but not-yet-disclosed vaccine, in order to determine whether BriLife “is at least as good as the licensed vaccine,” Javitt said.
“We’re also looking to make sure the vaccine is producing the right amount of antibodies [against the virus],” he added.
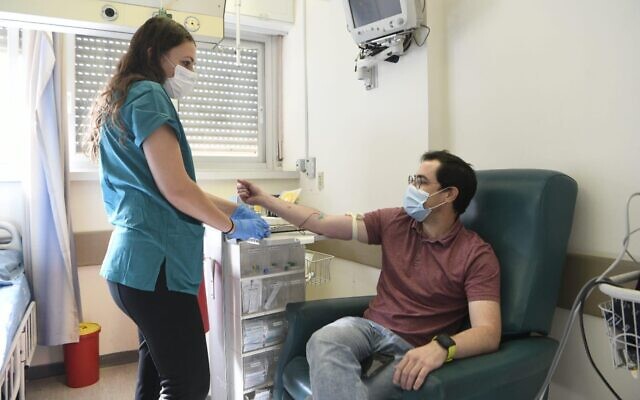
Anar Ottolenghi receives a dose of coronavirus vaccine developed by the Israel Institute for Biological Research at Jerusalem’s Hadassah Ein Kerem Hospital, on November 1, 2020 (Courtesy)
On this point, BriLife may have an advantage.
Whereas the world has been seeing waning effectiveness of the two-dose Pfizer/BioNtech vaccine after six months — a fact that prompted Israel to launch a booster campaign in late July — the IIBR released the results of an initial, small study that showed BriLife, given at a high dose, provides longer-term protection.
Some 200 volunteers who received the highest dosage of the vaccine were notified that they did not need a third dose of the vaccine, as their protection remained high six months after getting a second dose. These high doses were trialed in Israel and will soon be applied in Georgia, said Javitt.
The vaccine has also been tested against the highly contagious Delta variant and has shown “very good performance in lab models [animals] at a middle dose,” revealed Javitt. The institute was now waiting for final confirmation to begin testing in human trials, he added.

Jonathan Javitt. (Courtesy: Jonathan Javitt)
It is still a journey to go from trials to manufacturing millions of doses, said Javitt, adding that there was “a great deal of work to do,” but he appears confident in the final destination.
On the verge
For a brief moment in the spring of 2020, it appeared that Israel was on the verge of becoming a serious early player in the global race to produce a vaccine against COVID-19.
The secretive IIBR was already a few months into the research for a jab, having been tapped early on — in February 2020 — to begin urgently looking into what was then considered a mysterious pathogen alarming global health experts.
By late March, the institute said that it was making “significant progress” and would soon start testing on animals. Late in the summer, the center was ready to begin clinical trials with humans and that fall, it launched Phase I trials with 80 volunteers. But by winter, progress ran cold.
Almost a year later, the Israeli vaccine is still lagging way behind its international competitors — most notably Pfizer/BioNtech, which the Israeli government used to launch its mass vaccination campaign last December.
That campaign appears to have played an important part in the slowdown of the vaccine’s development and led to questions about the need to pursue a locally-made jab when other, promising candidates were already in late-stage development.

Director of the Institute of Biological Research, Prof. Shmuel Shapira, at the laboratory in Ness Ziona, on August 6, 2020. (Ariel Hermoni/Defense Ministry)
Professor Shmuel Shapira, the former head of the IIBR and driving force behind the efforts to develop an Israeli COVID-19 vaccine, claimed in a new book that heavy government interference, unexplained regulatory delays, and some level of “sabotage” were also at play.
Shapira stepped down this past May in a surprising turn of events that cast further doubt on the future of the inoculation initiative.
“I have documentation that proves government officials worked to delay our progress for months on end,” he recently told Israeli news site Ynet.
The IIBR “had an effective vaccine in July of 2020 and had to begin the regulatory process. That was postponed time and time again, while the daily morbidity rate was in the tens of thousands. That was not the time for bureaucracy,” he told the publication.
Two months after Shapira left the institute, NRx stepped in to continue the development of the vaccine and oversee its clinical trials. The company immediately announced that testing would start outside Israel, in Georgia and Ukraine.
The project appeared slow “because it went from [recording progress in a matter of] weeks to months.” But the technological aspect — the development of the vaccine itself — “was known to be achieved,” Javitt said.
The Israeli vaccine
Unlike Pfizer/BioNtech and Moderna’s mRNA (messenger RNA) vaccines, which are a type of jab that teaches our cells how to make a protein that triggers an immune response, the BriLife shot is a live-virus vaccine in which the spike protein of the vaccine appears to evolve in a manner consistent with the evolution of SARS-CoV-2, the virus that causes COVID-19 in nature.
The vaccine is based on a previous, FDA-approved vaccine platform that was further optimized by IIBR, NRx has said.
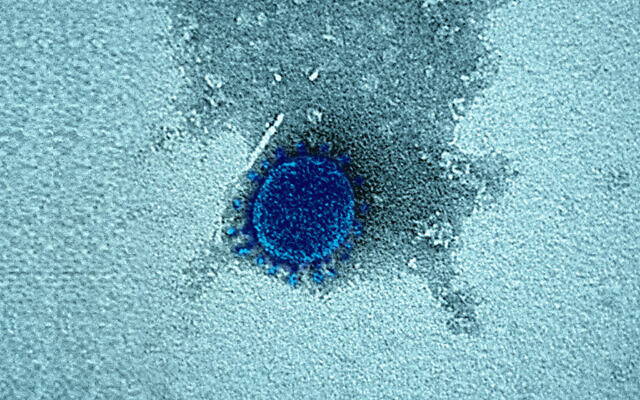
A novel coronavirus, blue, as seen in a photograph released by the Israel Institute for Biological Research, on October 25, 2020. (Defense Ministry)
The full results for BriLife have not been made public, so its efficacy against COVID-19 is less widely known than Pfizer’s and Moderna’s jabs. But Javitt said that once the company reviewed the data, “we became optimistic about the possibilities.”
A request by The Times of Israel for further information from the IIBR sent to the Defense Ministry was not answered by the time of publication.
NRx is now moving the project forward for a number of reasons, Javitt said: The waning effectiveness of the “first-generation vaccines,” the belief that BriLife may prove better at combatting COVID-19’s various variants, and a commitment to the State of Israel.
A promise to push forward with producing a vaccine for the people of Israel “was a condition for getting the contract” from the Defense Ministry, Javitt said. “It’s reasonable to say that the first priority is to the people of Israel.”
In addition, he said, “look around the world, people in hospitals dying. We need something that is going to keep people out of the intensive care unit. The antibodies produced by the [approved] vaccines are not as effective againt variants, only against original COVID. And there is no stopping the wave of variants.”

Magen David Adom workers wearing protective clothing evacuate a patient suspected of carrying the virus that causes COVID, at Hadassah Hospital Ein Karem in Jerusalem, on August 15, 2021. (Yonatan Sindel/Flash90)
Chaim Hurvitz, a former director of Israeli drugmaker Teva Pharmaceuticals and member of the board at NRx, said in July that “as the first generation COVID vaccines are increasingly challenged by rapid mutation of the coronavirus, we aim to develop a vaccine that can rapidly scale at low cost to serve the needs of both the developed and the developing world.”
Javitt also said that there were concerns about some of the known side effects of the tried-and-tested vaccines, such as myocarditis, an inflammation of the heart muscle.
With vaccines generally, the benefits outweigh the risks, but the equation is changing and benefits and risks must be balanced, he indicated. “We have an opportunity to generate a vaccine with fewer safety concerns.”
BriLife’s high dose also “wakes up the system which builds up immunity.”
Moreover, “the virus is uniquely capable of changing rapidly,” Javitt pointed out, noting the recent emergence of the Mu variant, a dominant strain in Colombia that is causing some concern after spreading to dozens of countries.
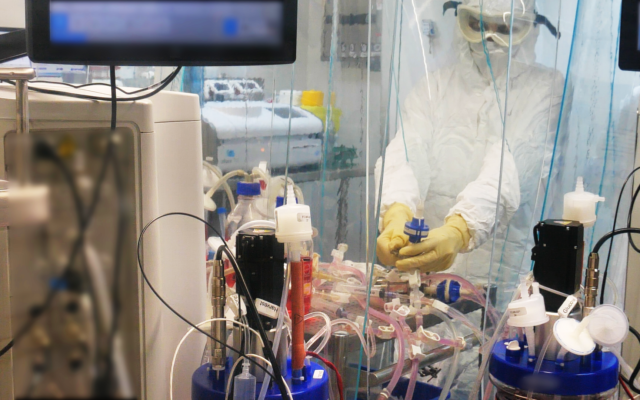
A scientist works in a laboratory in the Israel Institute for Biological Research, on October 25, 2020. (Defense Ministry)
“We need a proactive program like the [Israeli anti-missile system] Iron Dome, we need a plan of action,” he said. NRx would work closely with the IIBR to make sure to get ahead of new variants as they are detected worldwide, so consultations could begin on a vaccine design, Javitt said.
“The virus is evolving and mutating and posing new threats, the only hope of success would be to engage in a process to identify threats and respond,” he added.
The company was now focused on completing the clinical trials for BriLife and moving the process along. The price, once all regulatory approval is completed, will “be no higher than what is already out there,” Javitt said.
According to a Washington Post report last December, the US is paying $19.50 per Pfizer/BioNTech dose while the EU 27-country bloc is paying $14.76. The report cited Moderna vaccine prices as $15 per dose for the US and $18 per dose for the EU.
Israel, meanwhile, is said to have paid approximately $47 per two doses for the Pfizer-BioNtech and Moderna vaccines.
A boost toward commerzialition?
Earlier this month, NRx announced that former Mossad chief Tamir Pardo joined the company as a member of the board of directors.
The addition of Pardo, who served as head of the Mossad between 2011 and 2016, was meant to help the company push forward with the commercialization of the jab, the company indicated.
Pardo said in the announcement that he was “happy to continue serving the State of Israel,” and that the “success of blue-and-white [Israeli] developments, especially in the fight against the coronavirus, would be a success for everyone.”

Former Mossad chief Tamir Pardo. (Courtesy)
Javitt praised the former Mossad leader’s “extensive background in global security and bio-security and the esteem in which he’s held by leaders.”
Pardo’s “knowledge and experience in the international field will constitute a significant breakthrough in the distribution of the vaccine worldwide,” Javitt said.
NRx is also separately running trials for two pharmaceuticals. One is for NRX-101, a proposed drug to treat bipolar depression in patients with suicidal ideation. The company is still in the recruiting phase. A second study is for Zyesami (aviptadil), NRx’s proposed treatment for respiratory distress brought on by COVID-19 infection, currently in Phase IIb/III clinical trial in the US. The Times of Israel previously reported on this drug last year.
“We anticipate it will be beneficial against severe respiratory disease,” Javitt said.
"though" - Google News
September 20, 2021 at 05:30PM
https://ift.tt/3lFEjFi
Though lagging behind, Israel’s COVID-19 jab hopes to ‘find its place in market’ - The Times of Israel
"though" - Google News
https://ift.tt/2FnFft4
https://ift.tt/3dmAmQf
Bagikan Berita Ini

















0 Response to "Though lagging behind, Israel’s COVID-19 jab hopes to ‘find its place in market’ - The Times of Israel"
Post a Comment